The nitrilimine–alkene cycloaddition is an ultra rapid click reaction†
Abstract
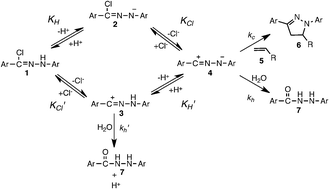
The transient formation of nitrilimine in aqueous conditions is greatly influenced by pH and chloride. In basic conditions (pH 10) with no chloride, a diarylnitrilimine precursor readily ionizes to form diarylnitrilimine that reacts almost instantly with an acrylamide-containing protein and fluorescently labels it.

 Please wait while we load your content...
Please wait while we load your content...