Catalytic asymmetric conjugate addition of terminal alkynes to β-trifluoromethyl α,β-enones†
Abstract
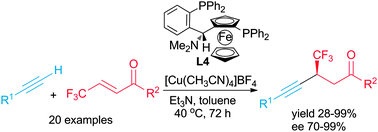
The first enantioselective conjugate alkynylation of β-trifluoromethyl α,β-enones using terminal alkynes and a taniaphos–Cu(I) complex as catalyst is described. Ketones bearing a trifluoromethylated propargylic chiral centre in the β-position were obtained with good yields and high enantiomeric excesses (up to 99%).

 Please wait while we load your content...
Please wait while we load your content...