Intermolecular (4+3) cycloadditions of aziridinyl enolsilanes†
Abstract
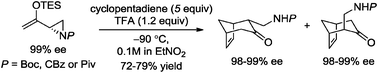
Upon activation by strong Brønsted acids, aziridinyl enolsilanes undergo (4+3) cycloadditions with dienes to afford aminoalkylated cycloheptenones as products. The use of a highly polar medium such as nitroalkane facilitates high cycloaddition yields of up to 99%. Optically pure aziridinyl enolsilanes react to yield (4+3) cycloadducts with up to 99% ee.

 Please wait while we load your content...
Please wait while we load your content...