Asymmetric synthesis of 3,4-annulated indoles through an organocatalytic cascade approach†
Abstract
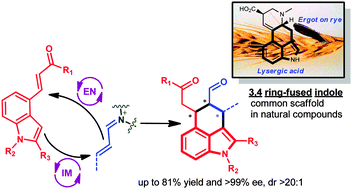
Indoles bearing Michael acceptors at the 4-position were engaged in organocatalytic enantioselective cascade reactions with enals. Careful optimisation of the reaction parameters overcame the inherent low reactivity of these substrates, rendering 3,4-ring fused indoles in good yields, excellent enantioselectivities and as single diastereoisomers.

 Please wait while we load your content...
Please wait while we load your content...