Highly enantioselective hydrogenation of 2-substituted-2-alkenols catalysed by a ChenPhos–Rh complex†
Abstract
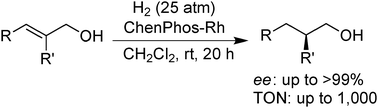
Highly enantioselective hydrogenation of a variety of 2-substituted-2-alkenols has been achieved using a ChenPhos–Rh complex as catalyst, giving ≥99% ee for most substrates. Optically active antifungal agent amorolfine was first synthesised using hydrogenation as the key step.

 Please wait while we load your content...
Please wait while we load your content...