Facilitated Li+ ion transfer across the water/1,2-dichloroethane interface by the solvation effect†
Abstract
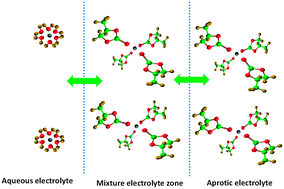
We demonstrate that the solvation effect can be the driving force for ion transfer across the water/1,2-dichloroethane interface. Voltammetric behaviours of facilitated Li+ ion transfer by the solvents of lithium-based batteries are investigated, which is valuable for the dual-electrolyte Li–air batteries, but also for the ion detection, separation and extraction.

 Please wait while we load your content...
Please wait while we load your content...