Enantioselective Black rearrangement catalyzed by chiral bicyclic imidazole†
Abstract
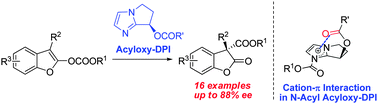
A newly developed chiral imidazole nucleophilic catalyst, Acyloxy-DPI, was readily prepared and successfully applied to the enantioselective Black rearrangement with up to 88% ee for a wide range of substrates possessing different substituted groups. A plausible mechanism for the high enantioselectivity was proposed.

 Please wait while we load your content...
Please wait while we load your content...