Base-catalyzed bicyclization of dialkyl glutaconates with cinnamoylacetamides: a synthetic strategy for isoquinolinedione derivatives†
Abstract
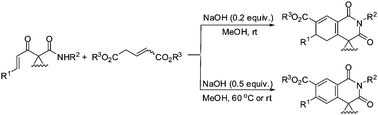
We report here that polysubstituted dihydroisoquinolones and isoquinolones can be constructed by the one-pot reaction of the readily available acyclic α,β-unsaturated carbonyl precursors and dialkyl glutaconates under mild basic conditions (1–45 min for the former vs. 1–6 h for the latter) via the domino process involving [3+3] annulation/intramolecular aza-cyclization.

 Please wait while we load your content...
Please wait while we load your content...