Palladium-catalyzed stereospecific cross-coupling of enantioenriched allylic alcohols with boronic acids†
Abstract
In the presence of 2.5 mol% Pd2(dba)3–TMEDA (1 : 4), a range of enantioenriched allylic alcohols smoothly coupled with boronic acids in a highly regioselective fashion with inversion of configuration to afford structurally diverse alkenes in good yields with perfect retention of ee.
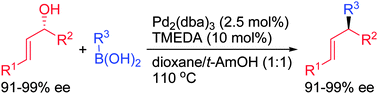

 Please wait while we load your content...
Please wait while we load your content...