Expanding dynamic kinetic protocols: transaminase-catalyzed synthesis of α-substituted β-amino ester derivatives†
Abstract
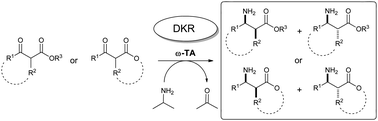
Several α-alkylated β-amino esters have been obtained via DKR processes employing a kit of transaminases and isopropylamine as an amino donor in aqueous medium under mild conditions. Thus, while acyclic α-alkyl-β-keto esters afforded excellent conversions and enantioselectivities, although usually low diastereoselectivities, using more constrained cyclic β-keto esters high to excellent inductions were obtained.

 Please wait while we load your content...
Please wait while we load your content...