Synthesis of inosine 6-phosphate diesters via phosphitylation of the carbonyl oxygen†
Abstract
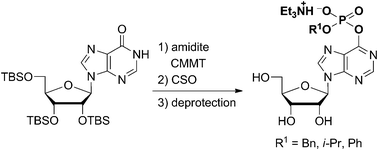
Inosine derivatives bearing a phosphodiester group at the O6-position of the nucleobase were synthesized via phosphitylation of the carbonyl oxygen using phosphoramidites activated by non-nucleophilic acidic activators.

 Please wait while we load your content...
Please wait while we load your content...