Evidence for the formation of a metal alkyl intermediate in the zinc mediated intramolecular hydroamination†
Abstract
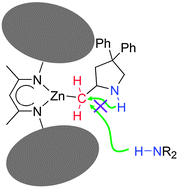
A diketiminato zinc amide complex, LZnNMe2 (1) [L = CH{(CMe)2(2,6-iPr2C6H3N)2}], was prepared and investigated for the catalytic hydroamination of 2,2-dimethylpent-4-en-1-amine. The reaction with the amino olefin resulted in a transamination reaction and the subsequent insertion of the olefin moiety into the metal–amide bond. However, the reaction stopped at this point providing access to the metal alkyl intermediate, LZnR (R = N![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) H–CH2–CPh2–CH2–C
H–CH2–CPh2–CH2–C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) H–) (2). The isolation of this primary insertion product further strengthens the widely accepted mechanism for the intramolecular hydroamination.
H–) (2). The isolation of this primary insertion product further strengthens the widely accepted mechanism for the intramolecular hydroamination.

 Please wait while we load your content...
Please wait while we load your content...