An easily removable stereo-dictating group for enantioselective synthesis of propargylic amines†
Abstract
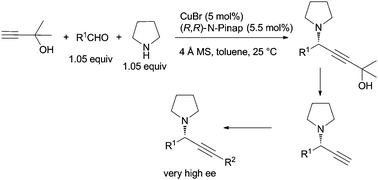
We report herein a CuBr-catalyzed three-component coupling of 2-methylbut-3-yn-2-ol, aldehydes and pyrrolidine or 1,2,3,4-tetrahydroisoquinoline leading to the corresponding chiral propargylamines in excellent enantiomeric excess (91 to >99% ee) and high yields (79–95% yield). The dimethylcarbinol unit in 2-methylbut-3-yn-2-ol, which may be easily removed at the later stage to regenerate a terminal alkyne unit for further elaboration, plays a very important role in ensuring high enantioselectivity. This protocol provides easy and very general access to different terminal and non-terminal tertiary propargylic amines.

 Please wait while we load your content...
Please wait while we load your content...