Lewis base assisted B–H bond redistribution in borazine and polyborazylene†
Abstract
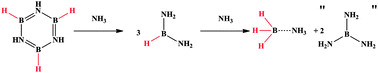
Lewis bases react with borazine and polyborazylene, yielding borane adducts. In the case of NH3 (l), ammonia-borane (AB) is formed and quantified using NMR spectroscopy against an internal standard. Calculations indicate that the formation of B(NH2)3 may provide the driving force of this redistribution. Given the complexity and expense of currently known spent AB regeneration pathways, it is suggested that this redistribution chemistry be used to recover AB and improve regeneration methods.

 Please wait while we load your content...
Please wait while we load your content...