Convergent de novo synthesis of vineomycinone B2methyl ester†
Abstract
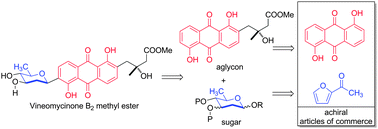
An efficient de novo synthesis of vineomycinone B2 methyl ester has been achieved. The longest linear route required only 14 steps from achiral commercially available starting materials (4.0% overall yield). The key transformations included the de novo asymmetric synthesis of two key fragments, which were joined by a convergent late stage Suzuki's

 Please wait while we load your content...
Please wait while we load your content...