“One pot” regiospecific synthesis of polysubstituted pyrroles from benzylamines and ynones under metal free conditions†
Abstract
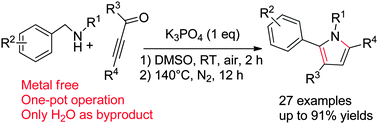
A convenient “one-pot” weak base-promoted synthesis of polysubstituted pyrroles has been developed from benzylamines and ynones. This transformation involves the Michael addition reaction and intramolecular condensation, which features high regioselectivity, high efficiency, environmental friendliness and metal free. A series of polysubstituted pyrroles were provided in up to 91% yield for 27 examples.

 Please wait while we load your content...
Please wait while we load your content...