Nickel-catalyzed manipulation of tertiary phosphinesvia highly selective C–P bond cleavage†
Abstract
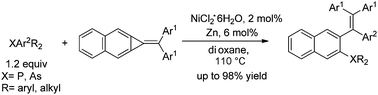
A catalytic cycle involving oxidative addition of nickel(0) with a carbon–carbon single bond in the three-membered ring of diarylmethylenecyclopropa[b]naphthalenes, highly selective cleavage of the C–P bond, and migration of the

 Please wait while we load your content...
Please wait while we load your content...