Functionalized heterocyclic scaffolds derived from Morita–Baylis–Hillman Acetates†
Abstract
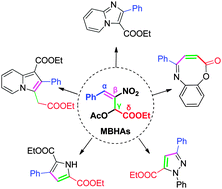
Five series of heterocycles with extraordinary structural diversity have been regiospecifically synthesized from the same Morita–Baylis–Hillman Acetates (MBHAs). All four potential electrophilic sites (α, β, γ, δ) of MBHAs are proved to be reactive.

 Please wait while we load your content...
Please wait while we load your content...