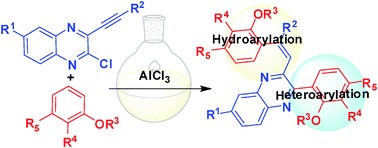
AlCl3-mediated hydroarylation–heteroarylation in a single pot: a direct access to densely functionalized olefins of pharmacological interest†
Abstract
An unprecedented AlCl3-mediated method has been developed involving aromatic C–H bond addition to an

 Please wait while we load your content...
Please wait while we load your content...