1,3-Dipolar cycloaddition of 4-platinumisochromenyliums with an olefin and tandem insertion into benzylic C–H bonds†
Abstract
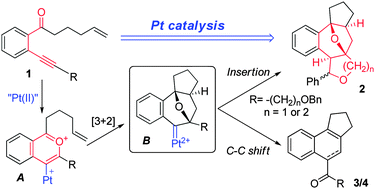
Isochromenylium-4-ylplatinum(II) species, generated from 1-(2-alkynylphenyl)hex-5-en-1-ones and Pt(II), reacted with a pendant

 Please wait while we load your content...
Please wait while we load your content...