Efficient synthesis of highly substituted pyrroles through a Pd(OCOCF3)2-catalyzed cascade reaction of 2-alkenal-1,3-dicarbonyl compounds with primary amines†
Abstract
We describe an unprecedented Pd(OCOCF3)2-catalyzed cascade process for the synthesis of highly functionalized 1,2,3,5-tetrasubstituted
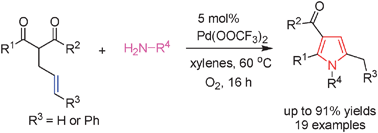

 Please wait while we load your content...
Please wait while we load your content...