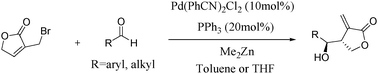
Pd-catalyzed diastereoselective allylation of aldehydes with 3-bromomethyl-5H-furan-2-one: stereoselective synthesis of β-(hydroxymethylaryl/alkyl)-α-methylene-γ-butyrolactones with a syn configuration†
Abstract
An efficient and diastereoselective Pd-catalyzed method of

 Please wait while we load your content...
Please wait while we load your content...