Five-fold symmetric penta-substituted corannulene with gelation properties and a liquid-crystalline phase†
Abstract
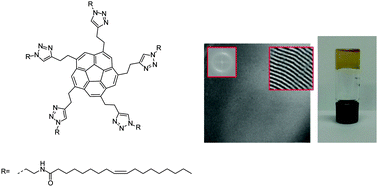
Five-fold symmetric substituted corannulene derivatives that display liquid-crystalline behavior and organogelation properties were prepared by coupling of N-azidoethyl

 Please wait while we load your content...
Please wait while we load your content...