Enantioselective synthesis of anti- and syn-β-hydroxy-α-phenyl carboxylates via boron-mediated asymmetric aldol reaction†
Abstract
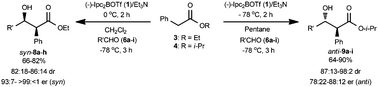
A reagent-controlled, diastereo- and enantioselective synthesis of anti- and syn-β-hydroxy-α-phenyl carboxylates has been achieved by the proper choice of

 Please wait while we load your content...
Please wait while we load your content...