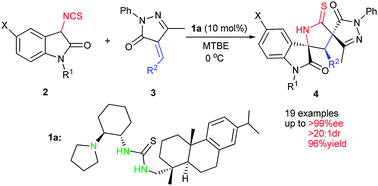
An asymmetric approach toward chiral multicyclic spirooxindoles from isothiocyanato oxindoles and unsaturated pyrazolones by a chiral tertiary amine thiourea catalyst†
Abstract
The first organocatalytic Michael–cyclization cascade reaction between isothiocyanato oxindoles and unsaturated pyrazolones has been developed. The multicyclic spiro[oxindole/

 Please wait while we load your content...
Please wait while we load your content...