Dual emissive borane–BODIPY dyads: molecular conformation control over electronic properties and fluorescence response towards fluoride ions†
Abstract
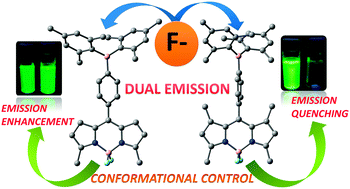
Facile synthesis of two new dimesitylboryl appended BODIPYs is reported. The two dyads have similar fluorescent

 Please wait while we load your content...
Please wait while we load your content...