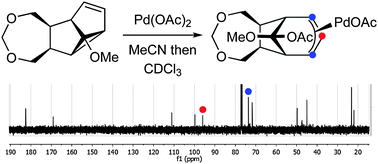
Confirming the existence of π-allyl-palladium intermediates during the reaction of meta photocycloadducts with palladium(ii) compounds†
Abstract
The transient existence of π-allyl-palladium intermediates formed by the reaction of Pd(OAc)2 and anisole-derived meta photocycloadducts has been demonstrated using

 Please wait while we load your content...
Please wait while we load your content...