Highly regioselective opening of zirconacyclopentadienes by remote coordination: concise synthesis of the furan core of the leupyrrins†
Abstract
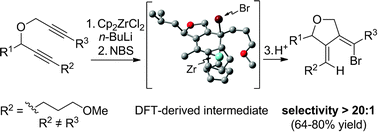
An efficient protocol for the highly regioselective opening of aliphatic zirconacyclopentadienes is reported. The one-pot process involves a

 Please wait while we load your content...
Please wait while we load your content...