Total syntheses of mitragynine, paynantheine and speciogynine via an enantioselective thiourea-catalysed Pictet–Spengler reaction†
Abstract
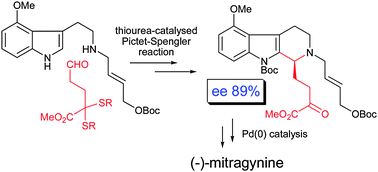
The pharmacologically interesting indole alkaloids (–)-mitragynine, (+)-paynantheine and (+)-speciogynine were synthesised in nine steps from

 Please wait while we load your content...
Please wait while we load your content...