Point-to-helical chirality transfer for a scalable and resolution-free synthesis of a helicenoidal DMAP organocatalyst†
Abstract
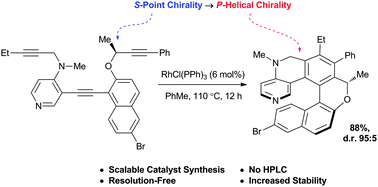
The synthesis of a second-generation [6]-helicenoidal DMAP organocatalyst is reported. The synthesis is reliant upon a highly diastereoselective Rh-catalysed [2 + 2 + 2]
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...