Phosphatase reactivity of a dicopper(ii) complex of a patellamide derivative – possible biological functions of cyclic pseudopeptides†
Abstract
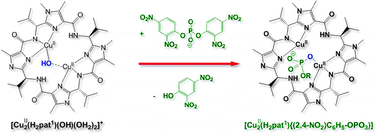
A possible biological function of cyclic pseudo-octapeptides is presented. The dinuclear copper(II) complex of a synthetic analogue ([Cu2(H2Pat1)(μ-OH)(OH2)2]) of the naturally occurring ascidiacyclamide is known to have a hydroxo-bridged dicopper(II) site which is able to catalytically transform CO2 into CO32−. This complex is shown here to function as a phosphatase mimic, suggesting that the so far unknown biological function of these

 Please wait while we load your content...
Please wait while we load your content...