Iron-catalysed cross-coupling of halohydrins with aryl aluminium reagents: a protecting-group-free strategy attaining remarkable rate enhancement and diastereoinduction†
Abstract
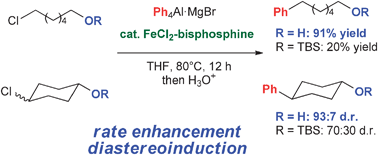
Non-protected halohydrins are cross-coupled with aryl aluminium reagents to produce aryl alkanols in the presence of the iron–bisphosphine

 Please wait while we load your content...
Please wait while we load your content...