Efficient, divergent synthesis of cryptophycin unit A analogues†
Abstract
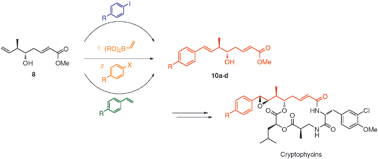
A flexible and divergent synthesis of cryptophycin unit A analogues is described. This method relies on iridium-catalysed stereo- and enantioselective crotylation and chemoselective one-pot oxidative olefination to access common intermediate 8. Heck,

 Please wait while we load your content...
Please wait while we load your content...