A cascade reaction of pyrrole-2-carbaldehyde substituted Morita–Baylis–Hillman adducts in the presence of tetrabutylammonium hydroxide or acetate to construct aza-heterocycles†
Abstract
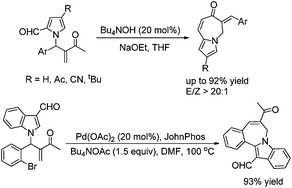
The phase-transfer catalyst promoted intramolecular transformation of pyrrole-2-carbaldehyde substituted Morita–Baylis–Hillman adducts has been disclosed, providing an efficient way to construct pyrrolo[1,2-a]azepin-7(6H)-one skeletons in moderate to good yields (up to 92%) under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...