An unexpected disproportional reaction of 2H-azirines giving (1E,3Z)-2-aza-1,3-dienes and aromatic nitriles in the presence of nickel catalysts†
Abstract
An unprecedented nickel-catalysed disproportional reaction of 2,3-diaryl-2H-azirines forming azabutadienes and aromatic
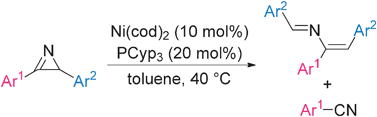

 Please wait while we load your content...
Please wait while we load your content...