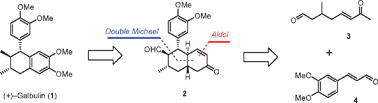
Enantioselective total synthesis of (+)-galbulinvia organocatalytic domino Michael–Michael–aldol condensation†‡
Abstract
A concise and practical enantioselective synthesis of
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...