Synthesis of polysubstituted 3-hydroxypyridines via the revisited hetero-Diels–Alder reaction of 5-alkoxyoxazoles with dienophiles†
Abstract
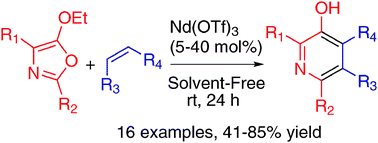
A general and single-step access to polysubstituted 3-hydroxypyridine scaffolds via hetero-Diels–Alder (HDA) reactions between readily prepared 5-ethoxyoxazoles and dienophiles is reported. The HDA reaction, run in the presence of Nd(OTf)3 at room temperature, was successfully applied to various 5-ethoxyoxazoles showing good functional group tolerance, and led to a straightforward process to obtain useful building-blocks.

 Please wait while we load your content...
Please wait while we load your content...