A Diels–Alder approach to the enantioselective construction of fluoromethylated stereogenic carbon centers†
Abstract
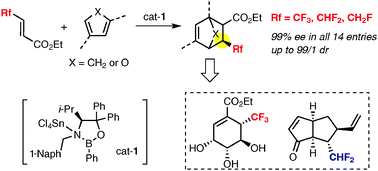
Highly enantioselective Diels–Alder reactions of β-fluoromethylacrylates were carried out in the presence of a Lewis acid activated chiral

 Please wait while we load your content...
Please wait while we load your content...