Supramolecular polymorphism of the 1 : 1 molecular salt (adamantane-1-carboxylate-3,5,7-tricarboxylic acid)·(hexamethylenetetraminium). A “failed” crystal engineering attempt†
Abstract
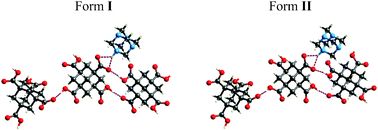
The tetrahedral arrangement of hydrogen bonding donor and acceptor groups is used to rationalise the design of a diamondoid network; however, a single proton transfer renders the four sites inequivalent, and results in two polymorphs of the title molecular salt utilizing similar intermolecular synthons.

 Please wait while we load your content...
Please wait while we load your content...