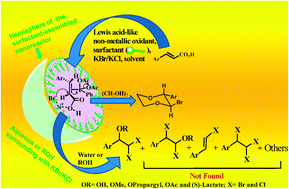
A fast and selective decarboxylative difunctionalization and cyclization for easy access to gem-dihalo alcohol, ether, ester and bromo-1,4-dioxane†
Abstract
A general strategy for fast decarboxylative difunctionalization to gem-dihalohydrin, gem-dihaloether, gem-dibromoester and cyclized

 Please wait while we load your content...
Please wait while we load your content...