Abstract
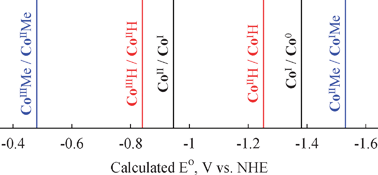
Our theoretical studies of the standard reduction potentials of the molecular complex [CoII(dmgBF2)2]0 (dmgBF2 = difluoro-boryldimethylglyoximate) in acetonitrile solution shed light on its electrocatalytic mechanism for hydrogen production. Three such mechanisms have been proposed, all proceeding through the formation of CoIIIH. Our results indicate that the mechanism involving a CoIIH intermediate is the most likely.
- This article is part of the themed collection: Artificial Photosynthesis

 Please wait while we load your content...
Please wait while we load your content...