Asymmetric Brønsted acid catalyzed carbonylactivation – organocatalytic domino electrocyclization–halogenation reaction†
Abstract
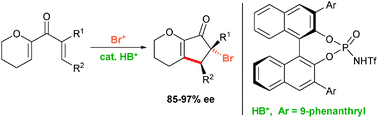
A highly efficient Brønsted acid catalyzed enantioselective Nazarov cyclization–bromination reaction has been developed. The protocol gives access to highly functionalized trans-4,5-substituted 5-bromocyclopentenone derivatives in good yields and with excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...