A general and efficient approach to 2H-indazoles and 1H-pyrazoles through copper-catalyzed intramolecular N–N bond formation under mild conditions†
Abstract
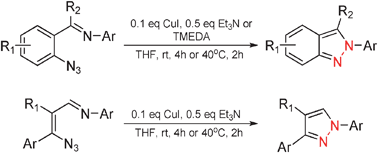
A new efficient copper-catalyzed intramolecular amination reaction has been developed to readily synthesise a wide variety of multi-substituted 2H-indazole and 1H-pyrazole derivatives from easily accessible starting materials under mild conditions. A highly selective

 Please wait while we load your content...
Please wait while we load your content...