Total syntheses of (Sp)-(+)- and (Rp)-(−)-spiniferin-1, a pair of unusual natural products with planar chirality†
Abstract
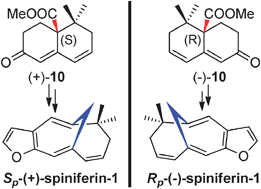
(Sp)-(+)-Spiniferin-1 and (Rp)-(−)-spiniferin-1, a pair of unusual marine natural products with planar chirality, were firstly synthesized via a polyfluoroalkanosulfonyl fluoride induced homoallylic carbocation rearrangement reaction. The chiral resolution and palladium-catalyzed β-H elimination of allylic

 Please wait while we load your content...
Please wait while we load your content...