(E,E)-1,5-Cyclooctadiene: a small and fast click-chemistry multitalent†
Abstract
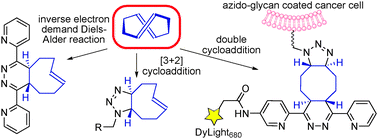
Two in one—We show here that the highly strained trans,trans-diolefin (E,E)-1,5-cyclooctadiene can perform efficiently two different click reactions at fast reaction rates. It is capable of first undergoing [3+2] cycloadditions with 1,3-dipoles at a reaction rate comparable to that of strained cyclooctynes. The resulting cycloadduct can then perform a much faster inverse-electron-demand Diels–Alder reaction with

 Please wait while we load your content...
Please wait while we load your content...