Solvent controlled mechanistic dichotomy in a Au(iii)-catalyzed, heterocyclization triggered, Nazarov reaction†‡
Abstract
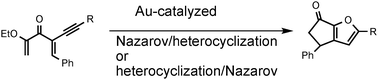
Tandem Au(III)-catalyzed heterocyclization/Nazarov cyclizations leading to substituted carbocycle fused
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...