Highly diastereo- and enantio-selective epoxidation of secondary allylic alcohols catalyzed by styrene monooxygenase†
Abstract
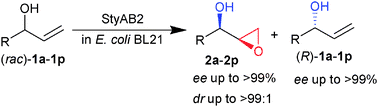
Enantiomerically enriched glycidol derivatives with contiguous stereogenic centers were obtained in a highly diastereo- and enantio-selective

 Please wait while we load your content...
Please wait while we load your content...