Direct asymmetric bromination of aldehydes catalyzed by a binaphthyl-based secondary amine: highly enantio- and diastereoselective one-pot synthesis of bromohydrins†
Abstract
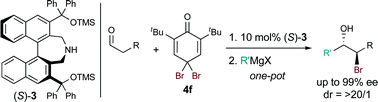
One-pot stereoselective synthesis of bromohydrins as a useful chiral building block was achieved by the reaction of

 Please wait while we load your content...
Please wait while we load your content...