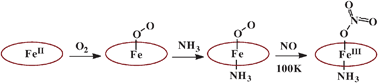
Hexacoordinate oxy-globin models Fe(Por)(NH3)(O2) react with NO to form only the nitrato analogs Fe(Por)(NH3)(η1-ONO2), even at ∼100 K†
Abstract
The oxy-globin models Fe(Por)(NH3)(O2), prepared by sequential reactions of O2 (18O2) and NH3 with thin porous layers of

 Please wait while we load your content...
Please wait while we load your content...