Electrochemical generation of silver acetylides from terminal alkynes with a Ag anode and integration into sequential Pd-catalysed coupling with arylboronic acids†
Abstract
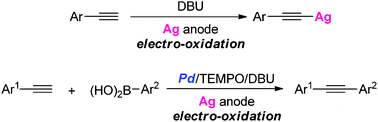
An electro-oxidative method for generating silver acetylides from acetylenes with a

 Please wait while we load your content...
Please wait while we load your content...